 Research Interests
Research Interests
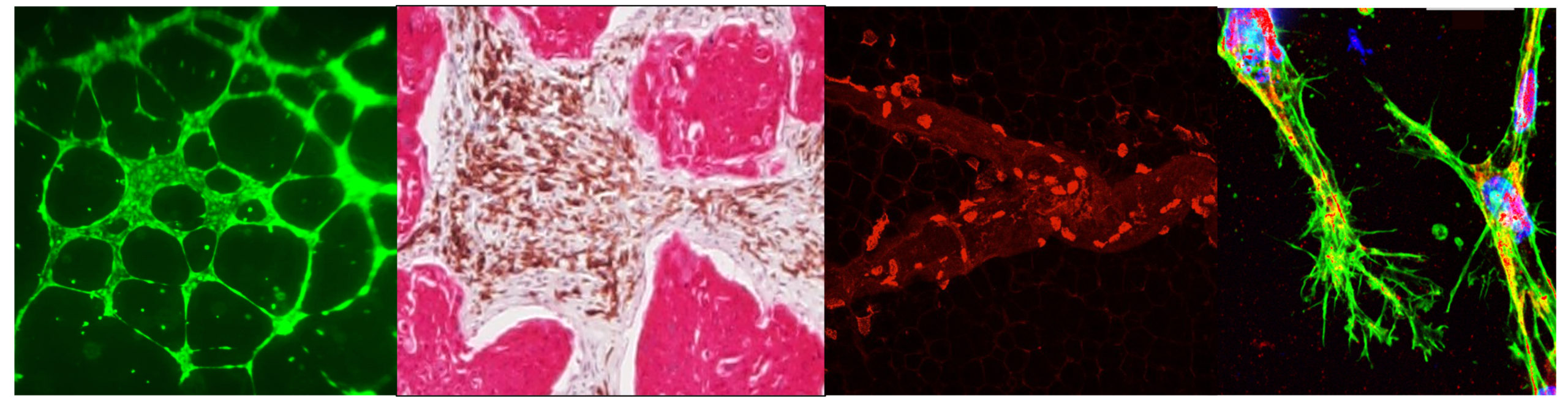
Lymphatics in Tumor Metastasis
Lymph node metastasis is a critical prognostic indicator of tumor progression and often associated with poor patient outcome. Presence of tumor cells in the lymph node is associated with tumor aggressiveness, yet the molecular mechanisms modulating tumor-lymphatic crosstalk remains grossly understudied. Further, expansion of the lymphatic network (or lymphangiogenesis) near a growing tumor plays a significant role in metastatic progression. Research in my lab, is focused on investigating how an inflammatory microenvironment modulates the tumor vasculature, promotes metabolic reprograming, endothelial to mesenchymal transition (EMT) mechanisms and enhances metastasis of tumors to the sentinel tumor draining nodes and to distant metastatic sites. Recent studies from the lab show that inflammation is a prime modulator of tumor-lymphatic crosstalk at the primary tumor bed and modulates remodeling of the tumor draining node to establish a pro-metastatic niche. Our lab employs interdisciplinary approaches from cell biology, immunobiology, physiology, bioengineering and biomechanics to address the fundamental alterations underlying lymphatic progression of different solid tumors that primarily metastasize through lymphatics. Current projects include:
-
Lymph Node metastasis of liver cancers (Cholangiocarcinoma and Hepatocellular Cancers):
We are investigating the roles of lymphatics in progression of inflammatory liver disease (such as cholestatic disease and NASH) to cancers such as cholangiocarcinoma and HCC. Cholangiocarcinoma is a rare and aggressive cancer that arises in the bile ducts. It is clinically silent and has a dismal prognosis. Tumor-associated growth of new lymphatic vessels (i.e. lymphangiogenesis) predicts unfavorable prognosis of CCA with tumor metastasis to the draining lymph nodes (LNs) as the primary indicator of tumor aggressiveness. Our CPRIT funded projects are primarily focused on a) understanding how lymphatic endothelial cells (LECs) reprogram the tumor cells to increase tumor growth and migration. Our recent data shows that specific chemokine-chemokine receptor interactions promote tumor-lymphatic interactions and enhance tumor promoting events in progression of liver cancer.
-
Development and identification of small molecule inhibitors targeted to lymph node metastasis:
We are currently developing novel combinatorial approaches to identify the efficacy of FDA approved targets on LN remodeling and growth of tumor associated lymphatic vessels. Specific check-point inhibitors show greater efficacy with tumor associated lymphatic vessels compared to blood vessels that directly defines patient specific response to therapy. Using single-cell RNA Seq along with bioinformatic and computational approaches we are developing a detailed transcriptomic profile of the LN microenvironment and also identification of specific tumor signatures that could act as a predictor for nodal metastasis.
-
Role of lymphatics in progression of therapeutic resistance in head and neck cancers:
HNSCC is the sixth most common cancer worldwide and despite advances in therapy has a 5 years survival probability of less than 50%. HNSCC often and preferentially migrates through lymphatics in the early stages that accounts for its poor prognosis. Studies from our lab has recently shown that entry of tumor cells into the lymphatics is tightly regulated by specific molecular interactions mediated by lymphatic endothelial cells (LECs) and also tumor cells acquire distinct vulnerabilities as a result of this tumor-LEC crosstalk that makes them resistant to traditional therapy. We are using bioengineered models, PDX models, Chip-Seq and liposomal targeting to dissect out the contributions of specific stromal elements in LN metastasis and drug resistance of HNSCC.
- Neuronal regulation of tumor metastasis: Sensory nerves release sensory neuropeptides and neurotransmitters from their peripheral terminals in tissues they innervate, and directly impact tumor progression by modulating systemic release of growth factors and mobilization of inflammatory cells. One such neuropeptide, Substance P, is a neuropeptide released by C-sensory fibers and that are closely associated with lymphatic vessels and implicated in multiple tumors. Modulation of Substance P and its receptors have emerged as clinical targets and we are currently investigating how SP mediated responses modulate immune recruitment in the primary tumor bed and impact nodal response. Our long-term research goal is to develop an understanding of neurotrophic factors regulating the tumor-lymphatic interactions in the biliary TME and neural regulation of metastatic LN, which will provide a foundation for the discovery of new pharmaceutical interventions for the prevention of CCA and HCC.
Lymphatics in Inflammatory Pathophysiology
The lymphatics through its function in immune surveillance and trafficking of immune cells are central to the process of immune response and modulation of inflammation. Lymphatic endothelial cells (LECs) have emerged as important players in modulating inflammatory responses, play key roles in resolution or progression of inflammation. Inflammation is a hallmark of several diseases making the role of lymphatics centre stage in many of these pathologies. The central focus of my lab is on the role of inflammation as a modulator of lymphatic associated pathologies
-
Lymphatics in Inflammatory Liver Disease:
Liver has a dense network of lymphatic vessels and recent studies implicate lymphatics play a significant role in maintenance of homeostasis and inflammation in the liver. Our most recent work shows that pathological levels of conjugated bile acids promote expansion of the lymphatic network and increases lymphangiogenesis in cholestatic models, and is associated with induction of oxidative stress pathways and directly regulates the vascular endothelial growth factor recepotor 3 (VEGFR3)- a key lymphangiogenic molecule. Further, we have demonstrated a significant expansion of the lymphatic network in inflammatory liver pathologies that is associated with progression of inflammation. The roles of lymphatic damage and functional dysfunction remains unknown in the context of liver pathology and we are currently investigating this functional correlation between lymphatic pump dysfunction and advancement of liver injury.
-
Role of microbiota dysbiosis in lymphatic inflammation associated pathologies:
Another focus of the lab is to investigate the role of inflammation and diet related changes in the gut microbiome (dysbiosis) and how specific microbiota metabolites impact lymphatic function, inflammatory response and affects diseases such as cancer, obesity, and metabolic disorders. This is a cross-collaborative research program funded by American Heart Association using molecular biology, immunology and metabolomics approaches with researchers in Chemical Engineering, Microbial Pathogenesis & immunology and Veterinary Medicine at Texas A&M. Specifically we are trying to determine a) Role of Tryptophan metabolites in lymphatic inflammation associated pathologies b) Effect of gender differences and epigenetic mechanisms in Sepsis and c) effect of microbiome on lymphatic-tumor crosstalk.