Our lab studies various herpesviruses but the major focus of our research is on the study of cytomegalovirus. Human cytomegalovirus (HCMV or Human herpesvirus 5) is a large DNA virus (>235 kb) that belongs to theBetaherpesvirinae genus of the Herpesviridaefamily. Primary HCMV infection in immunocompetent individuals is usually benign but establishes a lifelong latent state. Immune suppressed transplant recipients or AIDS patients are particularly susceptible to life-threatening end-organ disease. The other vulnerable population is the developing fetus in utero. During pregnancy, the vertical transmission of the virus across the placenta to the fetus (congenital infection) can lead to serious symptomatic disease in newborns that include mental retardation and deafness. Congenital CMV is the leading cause of mental retardation/deafness in newborns with over 5,000 children each year in the US. HCMV is also considered to be a contributing factor to vascular disease and specific cancers (eg. glioblastoma).
An Institute of Medicine report placed a vaccine to cytomegalovirus as a high priority for vaccines for the 21st century and various vaccine strategies are being investigated. An alternative intervention therapy, in lieu of a vaccine against HCMV, is the use of antivirals. Licensed antivirals are available for the treatment of transplant and AIDS patients. These drugs act at late stages of the virus life cycle and can lead to the development of resistant strains. There is an additional risk to the fetus of toxic side effects from extended drug therapy. Importantly, we have recently demonstrated in a novel humanized chimeric animal model that antiviral ganciclovir (the most commonly used HCMV antiviral for transplant patients) will reduce but not prevent congenital infection. Consequently, a vaccine against HCMV would be the best intervention strategy.
Any proposed intervention therapy for HCMV should be evaluated in a pre-clinical animal model. HCMV is extremely species-specific, making direct study of infection in animal models untenable. The guinea pig is unique as the only small animal model for congenital CMV infection. We use this model in our studies into the pathogenicity of the virus as well as the development of intervention strategies against congenital infection. Importantly, the guinea pig animal genome was recently sequenced at 7x coverage which enables the easy development of new reagents for the guinea pig (http://www.ensembl.org/Cavia_porcellus/Info/Index). Our research uses guinea pig cytomegalovirus (GPCMV) which is specific for the guinea pig and GPCMV infection closely parallels the HCMV infection in humans.
(1) Development of novel vaccine intervention strategies against congenital CMV. We are currently investigating various vaccines approaches against GPCMV and congenital infection. These approaches focus on neutralizing antibody target antigens (eg. viral glycoproteins) as well as T cell targets (eg. pp65 and IE1 homologs). In one novel approach, we are using a live defective viral vaccine approach to induce natural immunity with a virus that can infect cells and express the entire array of viral antigens but lacks the ability to produce infectious virus. Consequently, this approach is highly immunogenic but relatively safe. Our phase I studies provided high protection against congenital infection and we are currently developing phase II viral vaccines that encode additional important viral antigens related to viral infection of epithelial cells.
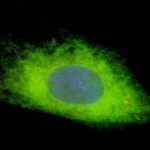
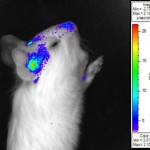
(2) Studies into CMV tropism, pathogenicity and congenital infection. We are using infectious BAC clones of GPCMV to mutate various viral genes to establish specific genes/ viral proteins associated with: essential function; virulence specific tissue tropism; or immune evasion strategies of the virus. Of specific interest are factors that influence viral invasion of the placenta during pregnancy and infection of the fetal brain. These studies employ novel non-invasive strategies such as bioluminescence imaging to track the virus in real time in the animal model.
(3) Impact of the pro-inflammatory response of the placenta to CMV infection. The inflammatory innate immune response mediated via toll-like receptors (TLRs) expressed on key placental cells (trophoblasts) is potentially an important aspect of controlling placental invasion/ congenital infection by both bacteria and viruses. CMV is known to be recognized by multiple TLRs but the placental TLR mediated response to CMV, or other pathogens, is poorly defined. The greatest likelihood of congenital CMV occurs during primary infection of a seronegative mother during pregnancy. In this situation, there is up to a 1:3 chance of transplacental infection to the fetus. In the absence of adaptive immune response to CMV, this rate of congenital infection might be perceived as lower than expected. Potentially, it implies that CMV transplacental infection is partially controlled by the innate immune response. It is our hypothesis that successful congenital infection is reliant upon the ability of CMV to efficiently enter and usurp TLR expressing trophoblast cells, an important component of the placental barrier and placental innate immunity.
These areas of research are being investigated by various novel cutting edge techniques in Molecular Biology, Virology and Immunology. Our research is currently funded by research grants from the National Institute of Health (NIH/ NIAID).