My laboratory studies the pathogenesis of diseases caused by Shiga toxins, a family of genetically and functionally related protein toxins expressed by the enteric pathogens Shigella dysenteriae type 1 and select serotypes ofEscherichia coli. Shiga toxin-producing E. coli have been in the news lately as ingestion of ground beef, spinach or well water contaminated with the toxin-producing organisms has resulted in widespread outbreaks of bloody diarrhea. Unfortunately, patients with these diarrheal diseases are at increased risk for developing life-threatening extra-intestinal complications including acute renal failure and neurological abnormalities. There are several research projects ongoing in the laboratory: (i) characterization of intracellular signaling pathways activated by Shiga toxins in macrophages leading to the increased expression of proinflammatory cytokines; (ii ) characterization of toxin- induced signaling pathways leading to apoptosis; (iii) examination of the roles of proinflammatory cytokines and apoptosis in pathogenesis using a murine model of Shiga toxin-induced renal damage; (iv) characterization of the global transcriptional response of human macrophages to Shiga toxins using microarray analysis; and (v) examination of the capacity of Shiga toxins to elicit the ER stress response.
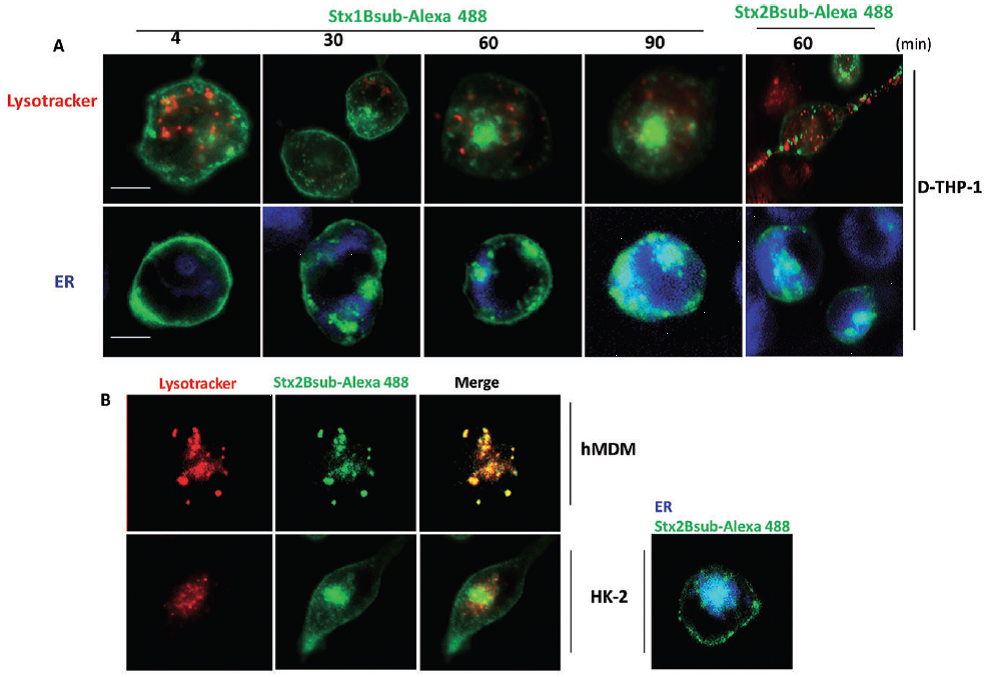
Stx1 B-subunit-Alexa488 and Stx2 B-subunit-Alexa488 trafficking in toxin-resistant and toxin-sensitive cells: A. Toxin-sensitive differentiated macrophage-like THP-1 cells (D-THP-1) were treated with 100 nM Lyso-Tracker (red) or 60 nM ER-Tracker (blue) Live Cell Staining dyes for detection of lysosomes and ER respectively. Subsequently, cells were stimulated with complete growth media containing 500 ng ml-1 Stx1 B-subunit-Alexa488 (Stx1Bsub-Alexa488) or Stx2 B-subunit-Alexa488 (Stx2Bsub-Alexa488; green) for the indicated times. B. Trafficking of Stx2Bsub-Alexa488 (green) to lysosomes and ER in toxin-resistant primary human monocyte-derived macrophages (hMDM) and toxin-sensitive HK-2 cells was detected using Lyso-Tracker (red; left panels) and ER-Tracker (blue; right panel) as described in (A). Cells were stimulated with complete growth media containing 500 ng ml-1 Stx1 B-subunit-Alexa488 (Stx1Bsub-Alexa488) for 10 min. A single confocal optical section through the middle of the majority of cells in the field of view was taken for red, blue or green emission channels simultaneously using a Stallion Digital Imaging Station. Images are representative of three independent experiments. All data within each experiment were collected at identical settings. The data suggest that Stx B-subunits rapidly translocate to the ER in toxin-sensitive cells, while Stx2 B-subunits traffic to lysosomes in toxin-resistant primary hMDM.
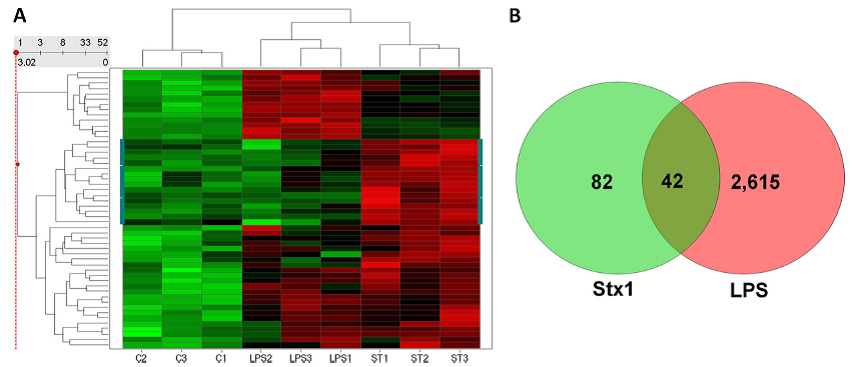
(A) Hierarchical clustering (performed using Spotfire DecisionSite) shows subsets of genes that are specifically upregulated in response to LPS or Stx1 (marked on the sides by a blue-green line) or are co-regulated by LPS and Stx1. Bright green indicates very low signal values, bright red represents very high signal values, and black represents median signal values. C, control (untreated); LPS, LPS-treated; ST, Stx1-treated cells. Each replicate is indicated by the numbers 1 to 3. Each of the sample types (C1 to 3, LPS1 to 3, and ST1 to 3) clustered together and apart from the other two sample types, and LPS- and Stx1-treated macrophages were more similar to each other than to untreated control cells. One subset of genes (indicated by the blue-green line on either side) was specifically upregulated in response to Stx1 treatment but not LPS treatment. (B) Venn diagram showing upregulated gene probes after Stx or LPS treat-ment. GeneSifter software was used to perform RMA normalization, followed by pairwise comparisons and Student t test with a Benjamini and Hochberg correction; the cutoffs used were a fold change of at least 1.5 and an adjusted P value of <0.05.